A number of times throughout this book, you’ve come across the terms “delayed stomach-emptying” and “gastroparesis.” As I explained in Chapter 2, elevated blood sugars for prolonged periods can impair the ability of nerves to function properly. It’s very common that the nerves that stimulate the muscular activity, enzyme secretion, and acid production essential to digestion function poorly in long-standing diabetes. These changes affect the stomach, the gut, or both. Dr. Richard McCullum, a noted authority on digestion, has said that if a diabetic has any other form of neuropathy (dry feet, reduced feeling in the toes, diminished reflexes, et cetera), he or she will also experience delayed or erratic digestion.
Slowed digestion can be fraught with unpleasant symptoms (rarely), or it may only be detectable when we review blood sugar profiles (commonly) or perform certain diagnostic tests. The picture is different for each of us. For more than twenty-five years, I suffered from many unpleasant symptoms myself. I eventually saw them taper off and vanish after thirteen years of essentially normal blood sugars. Some of the physical complaints possible (usually after meals) include burning along the midline of the chest (“heartburn”), belching, feeling full after a small meal (early satiety), bloating, nausea, vomiting, constipation, constipation alternating with diarrhea, cramps a few inches above the belly button, and an acid taste in the mouth.
GASTROPARESIS: CAUSES AND EFFECTS
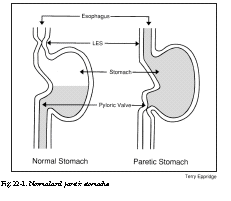 Most of these symptoms, as well as effects upon blood sugar, relate to delayed stomach-emptying. This condition is called gastroparesis diabeticorum, which translates from the Latin as “weak stomach of diabetics.” It is believed that the major cause of this condition is neuropathy (nerve impairment) of the vagus nerve. This nerve mediates many of the autonomic or regulatory functions of the body, including heart rate and digestion. In men, neuropathy of the vagus nerve can also lead to difficulty in achieving penile erections. To understand the effects of gastroparesis, refer to Figure 22-1.
Most of these symptoms, as well as effects upon blood sugar, relate to delayed stomach-emptying. This condition is called gastroparesis diabeticorum, which translates from the Latin as “weak stomach of diabetics.” It is believed that the major cause of this condition is neuropathy (nerve impairment) of the vagus nerve. This nerve mediates many of the autonomic or regulatory functions of the body, including heart rate and digestion. In men, neuropathy of the vagus nerve can also lead to difficulty in achieving penile erections. To understand the effects of gastroparesis, refer to Figure 22-1.
On the left is a representation of a normal stomach after a meal. The contents are emptying into the intestines, through the pylorus. The pyloric valve is wide open (relaxed). The lower esophageal sphincter (LES) is tightly closed, to prevent regurgitation of stomach contents. Not shown is the grinding and churning activity of the muscular walls of the normal stomach.
On the right is pictured a stomach with gastroparesis. The normal rhythmic motions of the stomach walls are absent. The pyloric valve is tightly closed, preventing the unloading of stomach contents. A tiny opening about the size of a pencil point may permit a small amount of fluid to dribble out. When the pyloric valve is in tight spasm, some of us can sometimes feel a sharp cramp above the belly button. Since the lower esophageal sphincter (LES in Figure 22-1) is relaxed or open, acidic stomach contents can back up into the esophagus (the tube that connects the throat to the stomach). This can cause a burning sensation along the midline of the chest, especially while the person is lying down. I have seen patients whose teeth were actually eroded over time by regurgitated stomach acid.
Because the stomach does not empty readily, one may feel full even after a small meal. In extreme cases, several meals accumulate and cause severe bloating. More commonly, however, you may have gastroparesis and not be aware of it. In mild cases, emptying may be slowed somewhat, but not enough to make you feel any different. Nevertheless, this can cause problems with blood sugar control. Consuming certain substances, such as tricyclic antidepressants, caffeine, fat, and alcohol, can further slow stomach-emptying and other digestive processes.
Some years ago, I received a letter from my friend Bob Anderson. His diabetic wife, Trish, who has since passed away, had been experiencing frequent loss of consciousness from severe hypoglycemia, caused by delayed digestion. His description of an endoscopic exam, when he was allowed to look through a flexible tube into Trish’s stomach and
gut, paints a graphic picture.
All this brings me to today’s endoscopy exam. I watched through the scope and for the first time, I now understand what you have been saying about diabetic gastroparesis. Not until I viewed the inside of the duodenum did I understand the catastrophic effect of 33 years of diabetes upon the internal organs. There was almost no muscle action apparent to move food out of the stomach. It appeared as a very relaxed smooth-sided tube instead of having muscular ridges ringing the passage. I suppose a picture is worth a thousand words. Diabetic neuropathy is more than a manifestation of a tilting gait, blindness, and other easily observable presentations; it wrecks the whole system. This you well know. I am learning.
HOW DOES GASTROPARESIS AFFECT BLOOD SUGAR CONTROL?
Consider the individual who has very little phase I insulin release and must take fast-acting insulin or one of the older-type (sulfonylurea) or newer pancreas-provoking OHAs before each meal. If he were to take his medication and then skip the meal, his blood sugar would plummet.
When the stomach empties too slowly, it can have almost the same effect as skipping a meal. If we knew when the stomach would empty, we could delay the insulin shot or add some NPH insulin to the regular to slow down its action. The big problem with gastroparesis, however, is its unpredictability. We never know when, or how fast, the stomach will empty. If the pyloric valve is not in spasm, the stomach contents may empty partially within minutes and totally within 3 hours. On another occasion, when the valve is tightly closed, the stomach may remain loaded for days. Thus, blood sugar may plummet 1–2 hours after eating, and then rise very high, say 12 hours later, after emptying eventually occurs. It is this unpredictability that can make blood sugar control impossible if significant gastroparesis is ignored in people who take insulin (or the type of OHAs I don’t recommend) before meals.
For most type 2 diabetics, fortunately, even symptomatic gastroparesis may not grossly impede blood sugar control, because they may still produce some phase I and phase II insulin. They therefore may not require significant amounts of injected insulin to cover their lowcarbohydrate meals. Much of their insulin is produced in response to blood sugar elevation. Thus, if the stomach does not empty, only the low basal (fasting) levels of insulin are released, and hypoglycemia does not occur. Of course, the sulfonylurea and similar OHAs (which I don’t recommend) can cause hypoglycemia under such circumstances.
If the stomach empties continually but very slowly, the beta cells of most type 2s will produce insulin concurrently. Sometimes the stomach may empty suddenly, as the pyloric valve relaxes. This will produce a rapid blood sugar rise, caused by the sudden absorption of carbohydrate following the entrance of stomach contents into the small intestine. Most beta cells of type 2 patients then cannot counter rapidly enough. Eventually, however, insulin release catches up and blood sugar drops to normal, if a reasonable regimen is followed. If your supper doesn’t fully leave your stomach before you sleep, you may awaken with a high morning blood sugar due to emptying overnight, even though your bedtime blood sugar was low or normal.
In any event, if you do not require insulin or use a sulfonylurea type OHA before meals, there is no hazard of hypoglycemia due to delayed stomach-emptying. This assumes that any long-acting insulin or sulfonylurea is administered in doses that cover only the fasting state, as discussed in prior chapters. The traditional use of large doses of these medications, meant to cover both the fasting and fed states, brings with it the hazard of postprandial hypoglycemia when gastroparesis is present.




